SOPHIA ANTIPOLIS, France – September 03, 2024 │ The quarterly report for the Q2 2024 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. What’s new this quarter?
Q2 2024 patenting activity maintains steady pace with notable new granted patents in key territories
The latest data on mRNA-related patenting activity indicates a stable trend, consistent with the previous quarter (see Q1 2024 press release for details). A thorough analysis of newly granted patents across monitored territories, i.e., United States (US), European Patent Office (EP), Japan (JP), and South Korea (KR), reveals a total of 31 inventions granted for the first time within this geographical scope. This includes 13 granted patents in Japan, 11 in the US, 4 in Europe, and 3 in South Korea, demonstrating ongoing innovation and protection in this critical biotechnology field.
As seen in recent years, mRNA delivery remains the most represented technological segment, accounting for more than 50% of the newly granted patents. Among the key players this quarter, SANOFI leads with four new patents, predominantly in the delivery sector, including three related to lipid nanoparticle (LNP) components and one involving the encapsulation process. This confirms the position of SANOFI in the therapeutic mRNA area, thanks to the acquisition of TRANSLATE BIO (see our last insight on 2023 patenting activity). ACUITAS THERAPEUTICS has secured three patents, including two on lipids for LNP and one on mRNA vaccine in collaboration with PENN UNIVERSITY. BIONTECH continues to strengthen its position in oncology with three newly granted patents focused on cancer therapies. For more details on mRNA cancer therapies, see our previous Report.
This ongoing patenting activity underscores the continued growth and innovation within the mRNA space, with a strong emphasis on improving delivery mechanisms and expanding therapeutic applications.
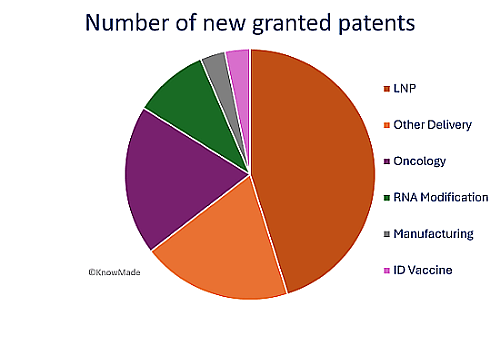
Figure 1: Q2 2024 Technological segmentation of newly granted patents
Q2 2024 Focus on a newcomer developing therapeutic circular RNA
Founded in 2014 in Beijing’s Zhongguancun Life Science Park, SYNGENTECH is a Chinese company dedicated to advancing synthetic biology for the development of novel gene therapies targeting cancer and infectious diseases. Notably, the company’s flagship product, SynOV1.1, a targeted tumor gene therapy drug, has gained approval from both the FDA and NMPA for Phase I clinical trials. SYNGENTECH has since expanded its research and development efforts into mRNA and circular RNA (circRNA).
Within its patent portfolio, SYNGENTECH has focused significantly on circular mRNA therapeutics, a promising new avenue in gene therapy. The company has five Chinese patent applications (published between June 2024 and July 2024), related to circular mRNA vaccines against feline infectious peritonitis virus (FIPV) and one granted patent for the mRNA cyclization process. Feline Infectious Peritonitis (FIP) is a serious and often fatal disease in cats, caused by some mutated strains of the feline coronavirus (FCoV). FIP manifests in two forms: the “wet” form, characterized by fluid accumulation, and the “dry” form, affecting the central nervous system. The disease is typically progressive and lethal without treatment, but recent advancements in veterinary medicine, including newly available therapies, offer hope for affected cats. Among these therapies, circular RNA vaccination presents distinct advantages (i.e., stability and immunogenicity of the circular mRNA) as discussed in our previous insight on this topic, see here.
SYNGENTECH has the potential to become a key player in the circular mRNA therapeutics market, particularly if it expands its currently China-focused patent portfolio. While the company’s five patent applications, related to circular mRNA vaccines for cat vaccination, are currently limited to China, they hold the potential to be extended to other territories, as other patent families held by SYNGENTECH. Should SYNGENTECH pursue international patent protection, it could significantly enhance its global influence in the rapidly evolving field of circular mRNA therapeutics. However, if the company chooses not to extend these patents, its impact may remain confined to the Chinese market. This scenario highlights the critical importance of monitoring mRNA patent activities to understand the intellectual property position and strategy of the different players in this fast-evolving context.
If you require additional information, please reach us at contact@knowmade.fr or with our contact form.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
