SOPHIA ANTIPOLIS, France – November 04, 2024 │ The last quarterly report for the 2024 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics.
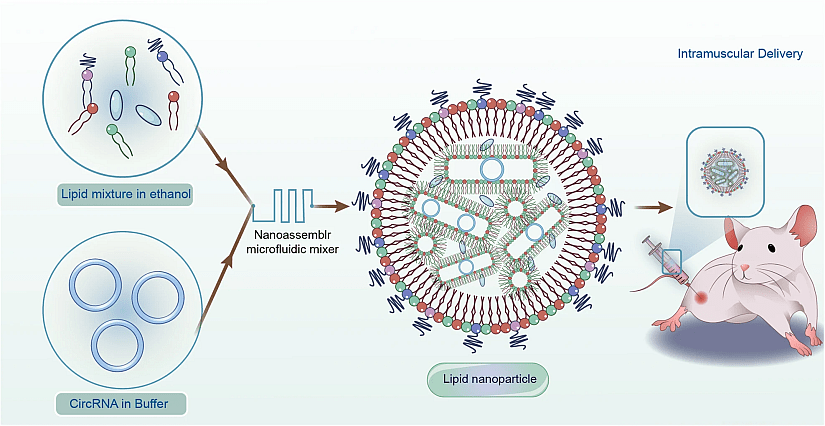
Diagram of LNP-Based Delivery for circRNA Vaccine (image from Niu et al., 2023).
Circular RNAs present advantages over conventional therapeutics mRNAs
Circular RNA (circRNA) is a unique type of single-stranded RNA that, unlike traditional linear RNA, forms a continuous loop by connecting its 3’ and 5’ ends. This closed-loop structure was first identified in humans in 1991 but initially dismissed as a splicing error. However, recent research has highlighted its important roles in gene regulation, influencing various biological processes, including development, physiological responses, and even diseases like cancer. While many circRNAs are non-coding, some have demonstrated the ability to encode proteins through mechanisms like internal ribosome entry sites (IRESs) and cap-independent translation, which opens up novel therapeutic applications, especially in fields targeting complex gene expression pathways and immune system modulation.
One of circRNA’s primary advantages for therapeutic use, including vaccine and gene therapy applications, is its stability. Unlike linear RNA, which is vulnerable to degradation by RNases, circRNA’s closed-loop structure provides natural resistance, enabling it to remain active within cells for extended periods. This structural stability means circRNA-based vaccines and therapies may require lower doses, reduce manufacturing costs and potentially lead to fewer side effects for patients. However, circRNA’s immunogenicity presents a dual-edged sword: it can be beneficial in vaccine development, where immune stimulation is desired, but may raise challenges in other applications, such as protein replacement therapies, where immune reactions could lead to adverse effects. Additionally, while circRNA can accumulate at high concentrations in cells, making it more efficient for sustained protein expression, it is unlikely to serve as a one-time treatment, requiring repeated doses over a patient’s lifetime.
A circular RNA vaccine prevents Zika virus infection
A recent scientific paper (Liu et al., 2024) explored the development of a circular RNA vaccine designed to protect against Zika virus (ZIKV) infection, addressing safety concerns related to cross-reactivity with dengue virus (DENV). Indeed, antibody-dependent enhancement (ADE) is a potential concern for the development of ZIKV vaccine. ADE is a phenomenon where antibodies, instead of protecting against a virus, inadvertently aid its entry into host cells, worsening the infection. In the context of Zika and dengue viruses, ADE occurs when antibodies generated against one virus (e.g., Zika) bind to but do not neutralize a related virus (e.g., dengue). These non-neutralizing antibodies can then facilitate the virus’s entry into immune cells via Fc receptors, potentially leading to more severe disease outcomes.
The strategy used to design the ZIKV was to combine advantages of circRNA and optimized coding sequences within the circRNA. Authors decided to fuse a ZIKV-specific epitope with high neutralizing potency but poor immunogenicity (EDIII) to human IgG1 Fc fragment (a known method for extending antigen half-life and for increasing the avidity for B-cell receptors through antigen dimerization) and to combine such circRNA with another circRNA encoding for NS1 (NS1-based vaccines have shown protective effects in several animal models without causing ADE). Two distinct formulations were tested, EN(LNP) which is a cocktail of LNPs encapsulating EDIII-Fc circRNA and NS1 circRNA separately, and EN(RNA) consisting of LNPs encapsulating a pre-mixed blend of EDIII-Fc and NS1 circRNAs. As illustrated in figure 1, a single-dose maternal immunization with either EN(LNP) or EN(RNA) fully protected the pups against the ZIKV-caused meningeal inflammation and cortical laminar necrosis, resulting in a pathological score of zero, whereas either EDIII-Fc circRNA or NS1 circRNA greatly reduced, but not eliminated, the ZIKV-cause brain damage.
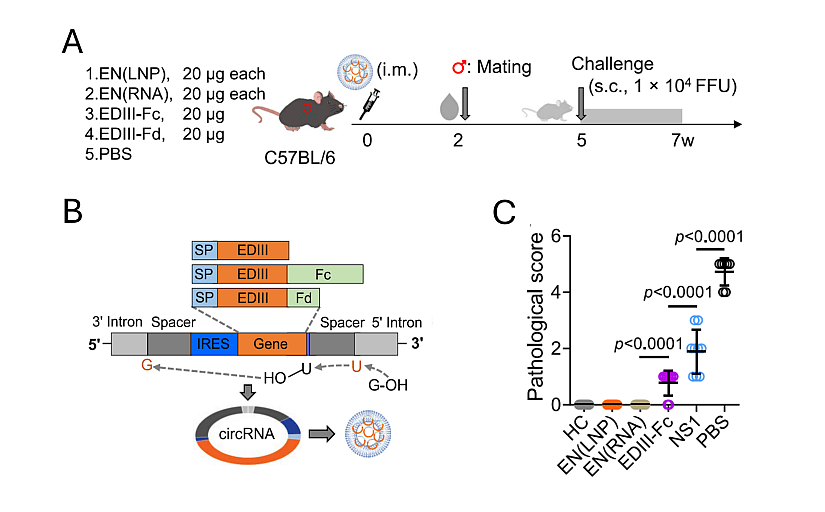
Figure 1: Design strategy for the production of ZIKV circular RNA vaccine.
A: Schematic diagram of maternal immunization and neonatal challenge model. Eight-week-old female C57BL/6 mice were i.m. immunized once with the indicated vaccines at 20 μg each circRNA per mouse or an equal volume of PBS. Immunized mice were mated at 2 weeks after immunization. After birth, 1-dayold pups were s.c. challenged with 1 × 104 FFU of ZIKV. Fifteen days later, pups were sacrificed.
B: Schematic diagram of RNA circularization. Coding sequences for EDIII, EDIII-Fc and EDIII-Fd were fused and cloned into circRNA plasmids. circRNAs were produced by IVT followed by circularization.
C: Pathological scores of neonatal brains. Meningeal lymphocyte infiltration and cortex laminar necrosis were scored in a single-blind manner.
From Liu et al., 2024.
Authors further concluded that the protective immunity elicited by the composition EN(LNP) (i.e., a cocktail of LNPs encapsulating EDIII-Fc circRNA and NS1 circRNA separately) is durable, as it is observed up to 11 weeks in mice, without causing ADE risk neither promoting DENV infection.
Hence, the circRNA vaccine detailed within this publication presents favorable results against Zika infection which is linked with severe neurological disorders, including Guillain-Barré syndrome in adults and microcephaly in newborn. Despite a decline in ZIKV transmission since 2017, several countries continue to report infection clusters. Currently, there are still no ZIKV vaccines approved for clinical use. Results obtained propose circRNA as a promising platform for safe and effective Zika virus, even flavivirus vaccine.
Circular RNAs are promising for mRNA vaccine development
Circular RNA represents a groundbreaking technology in therapeutic applications, offering potential advantages in stability and efficiency over traditional RNA approaches. Since 2022, KnowMade’s patent analysis has identified dynamic growth in this field, with key players like LARONDE, ORNA, and THERONA leading innovation (see our previous article for more information). The landscape evolved further when LARONDE merged with SENDA in 2023 to form SAIL Biomedicine, and ORNA, a leader with more than 25 patent families, acquired RENEGADE in 2024 to enhance its delivery capabilities. Indeed, RENEGADE’s strategic acquisition came with an interesting patent portfolio related to ionizable lipids and new LNP. This patent portfolio is detailed and analyzed in KnowMade’s article “RENAGADE Therapeutics unveiled new LNPs for delivering mRNA to extra-hepatic tissues”. This progression in circular RNA related landscape underscores the significance of delivery mechanisms and strategic partnerships in advancing circRNA therapies.
To keep up with these developments, KnowMade offers a comprehensive patent monitoring service that enables stakeholders to track trends and competitive moves in circRNA field. In 2023, 40 new patent families related to circRNA were published or granted, and this momentum continued into 2024, with additional new entrants like SYNGENTECH entering the field. SYNGENTECH’s portfolio focuses on circular mRNA vaccines, including patent applications targeting feline infectious peritonitis virus (FIPV), highlights the broadening applications of circRNA (see Q2 2024 Press Release for more details). KnowMade’s service allows companies and researchers to survey such advancements in real-time, providing insights into emerging trends and new entrants in this rapidly evolving therapeutic area.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
