SOPHIA ANTIPOLIS, France – November 05, 2024 │ The last quarterly report for the 2024 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics.
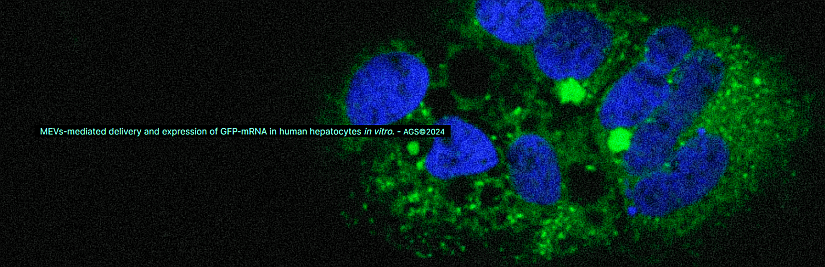
Image from AGS web site (https://www.ags-tx.com/gallery).
AGS Therapeutics: Pioneering Microalgae-Based Drug Delivery for Therapeutic mRNA
AGS therapeutic, a French newcomer to follow
Identified as a newcomer in January 2023, with an invention related to the mRNA delivery segment by the Therapeutic mRNA patent monitor from KnowMade, with its first patent application (WO2023/001894), AGS is quickly expanding its intellectual property portfolio. The company’s mission to harness the power of Chlorella’s extracellular vesicles for safe and targeted drug delivery offers new possibilities for therapeutic mRNAs, and positions AGS as an important player to follow in this rapidly advancing field.
Founded in May 2020 in Paris, France, AGS Therapeutics is a biotechnology company focused on developing delivery systems for therapeutic modalities. Building on the research of Dr. Lionel Navarro, a leading researcher at the Institut de Biologie of the École Normale Supérieure, AGS Therapeutics has developed a drug delivery technology based on microalgae extracellular vesicles (MEVs) derived from the freshwater microalga, Chlorella vulgaris (see figure 1).
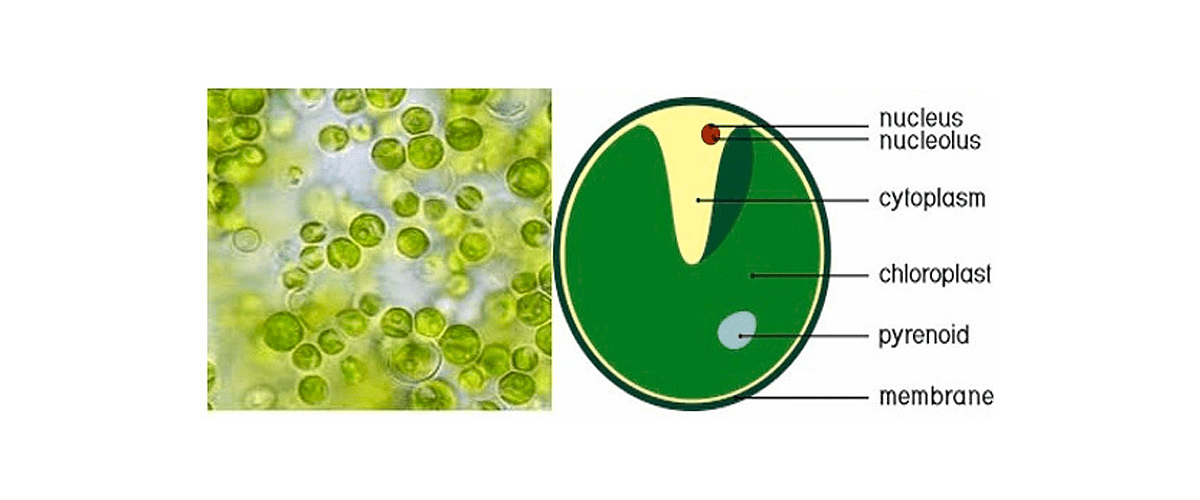
Figure 1: Structure of Chlorella vulgaris.
From Barghchi et al., 2023.
AGS Therapeutics is structured as a group of interconnected companies, with its subsidiary AGS-M specifically focused on optimizing the bioproduction and purification processes of MEVs. AGS-M plays a key role in the production of MEV batches for preclinical and clinical development, ensuring scalability and quality control for both AGS and its pharmaceutical partners.
In recent achievements, AGS Therapeutics has been recognized for its innovative use of MEVs as a delivery system for vaccines. The company won the prestigious Sanofi iDEA-iTech Award on October 8, 2024, for its breakthrough in intestinal mucosal vaccination via oral administration using its MEVs technology (see AGS press release). This comes on the heels of being named one of Business Worldwide Magazine’s “20 Most Innovative Companies to Watch” in 2023, solidifying AGS’s reputation as a leader in pioneering biopharmaceutical solutions that are transforming drug delivery and non-viral gene therapies (see the News).
Advantages of Microalgae Extracellular Vesicles (MEVs) in Therapeutic Delivery
According to AGS Therapeutics, MEVs offer a safe, targeted, and highly versatile delivery system for a range of innovative biologics, including mRNA. MEVs possess unique properties that set them apart from other delivery methods. Indeed, MEVs allow various delivery routes, such as oral delivery to reach the gut-associated lymphoid tissue and the spleen (bypassing the stomach), intranasal administration to access hard-to-reach areas like the brain (bypassing the blood-brain barrier), or via eye-drop instillation to reach retina (see figure 2, illustrating the versatility of administration). These characteristics offer new avenues for treating neurological and ocular conditions.
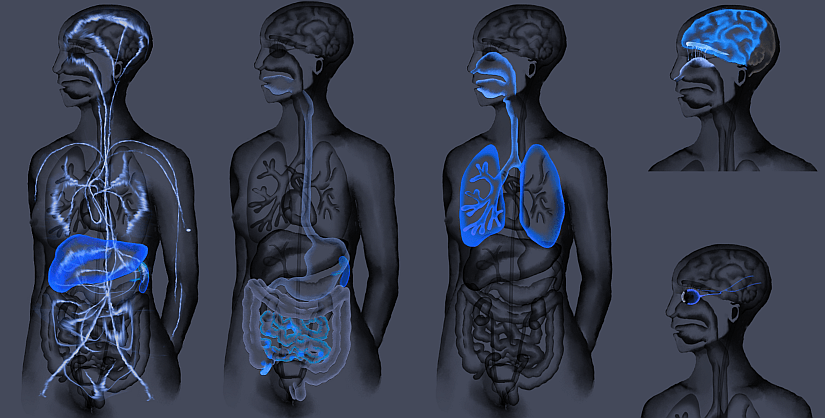
Figure 2: Delivery routes of MEVs.
From left to right, Intravenous delivery, oral delivery, inhalation, intranasal and ocular. From AGS pipeline.
MEVs are also highly scalable and eco-friendly. Produced naturally from Chlorella, a unicellular microalga recognized as safe by the FDA, MEVs are manufactured using simple, sustainable processes that require only water, minerals, and light. This ability to be produced in large quantities through scalable techniques, positions MEVs as a potentially revolutionary tool for drug delivery, particularly in mRNA-based therapeutics and vaccines.
AGS Therapeutics’ Technology
AGS develops MEVs from Chlorella vulgaris, a unicellular haploid alga that is a natural and efficient producer of extracellular vesicles. This alga has been consumed worldwide as a food supplement for decades; it is non-toxic, non-immunogenic, and can be cultured at large industrial scale at low cost. MEVs advantages are longer clearance rates than reported for mammalian EVs and an ability to cross natural body barriers (used for oral delivery, or specific lymphoid tissues delivery, or nose-to-brain delivery) that have not been attained with LNP and EVs of mammalian origin.
Therapeutic MEVs production and biodistribution
MEVs production by AGS is precisely described within the patent application WO2023/001894 “Extracellular vesicles from microalgae, their preparation, and uses” filed on 2022-07-20. Briefly, the production process comprises the step of microalgae culture in photobioreactor, MEVs isolation by centrifugation, MEVs purification by tangential flow filtration or by size exclusion, and finally MEVS loading with DNA, RNA, proteins or other small molecules (see the summary of the different methods in Table 1). MEVs’ loading can also be performed thanks to genetic modifications.
| Loading method | Loaded cargo |
| Passive and surfactant assisted | DNA and proteins |
| Freez-Thaw cycle | DNA, RNA and proteins |
| Sonication | DNA, RNA, proteins, and small molecules |
| Extrusion | DNA, RNA and proteins |
| Sonication / Extrusion | RNA and proteins |
| Electroporation | DNA, RNA and proteins |
Table 1: Summary MEVs loading methods detailed in WO2023/001894
MEVs enable various biodistribution reached thanks to different administration routes as described in figure 3. The AGS patent portfolio describes numerous experimental examples illustrating targeted mRNA expression. For instance, MEVs enable ocular mRNA expression following a single ocular topical administration in albino rabbits (WO2023/001894); they allow whole body mRNA expression after intra-tracheal administration in mice (WO2023/144127), or even the assessment of the capacity of MEVs as a mRNA vaccine delivery system after intramuscular (IM) administration (WO2024/194423).
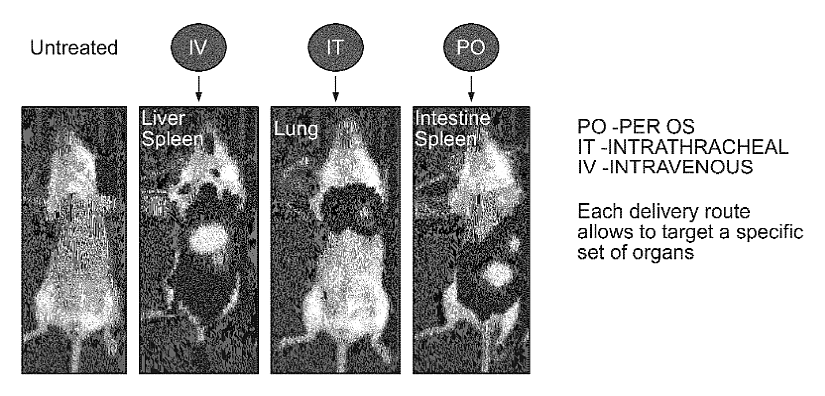
Figure 3: Representative patterns of biodistribution according to the route of administration, for the Intravenous, Intratracheal and Per os routes.
From WO2023/001894.
IP portfolio detail
AGS has strategically built a robust patent portfolio with six distinct patent families, all comprised of pending applications filed between 2022 and 2024, as described in table 2. Notably, AGS has pursued a comprehensive worldwide coverage strategy, evident through five international patent applications under the Patent Cooperation Treaty (PCT). This approach ensures potential market protection in multiple regions across the globe. The international applications, each accompanied by a positive written opinion from the International Searching Authority (ISA), highlight the strength and novelty of AGS’s technological advancements, placing the company in a favorable position within the global patent landscape. A standout among AGS’s patent families is WO2024/194423, the first filed patent family, where the ISA has confirmed that the claimed invention possesses both novelty and an inventive step, affirming the patent’s high potential for successful prosecution.
Each patent family targets unique aspects of therapeutic and diagnostic innovations. The first family introduces methods and compositions for delivering bioactive agents to specific organs or systems, including targeted delivery to the immune system, through isolated MEVs. The second family expands on this by developing MEV-based cell therapy compositions intended to treat diseases affecting specific organs and tissues, with a particular focus on brain-targeted drug delivery systems. The third family features genetically modified microalgae cell cultures that produce MEVs containing bioactive molecules, such as inhibitory RNA, with advanced drug delivery applications. In the fourth family, AGS has innovated diagnostic methods using intranasal administration of MEVs to deliver bioactive molecules to the brain via olfactory pathways, facilitating the detection and monitoring of neurological conditions. The fifth family provides compositions with exogenously loaded MEVs for diverse therapeutic, industrial, diagnostic, and cosmetic uses, while the sixth family presents vaccine compositions that utilize MEVs as carriers for immunomodulatory agents, showing promise for applications in immune response modulation.
| Publication Number | Subject of the invention | Current legal status | AGS Press release |
| WO2024/194423 | The use of MEVs in vaccines, including oral mucosal vaccination, and immunomodulation. | Pending Application (WO) | Oct.1, 2024 |
| WO2024/088808 | The use of MEVs to deliver biological agents to the brain by the intranasal route of administration | Pending Application (WO) | May 7, 2024 |
| WO2023/232976 | MEVs from Genetically Modified Microalgae Containing Endogenously Loaded Payloads | Pending Application (WO) | Jan. 17, 2024 |
| WO2023/144127 | Biodistribution and Uses of MEVs as Delivery System to Reach Difficult to Access Tissues | Pending Application (WO) | Aug. 7, 2023 |
| WO2023/001894 | Preparation and Uses of MEVs as a Universal Drug Delivery System | Pending Application (WO, EP, CA, AU, JP, CN) | May 8, 2023 |
| US20240173265 | A composition of isolated MEVs for animal administration In collaboration with Univ. de Nantes |
Pending Application (US) |
Table 2: Summary of AGS therapeutics’ patent portfolio
Conclusion
AGS Therapeutics exemplifies the transformative potential within the therapeutic mRNA landscape, leveraging microalgae-derived MEVs to pioneer novel, safe, and versatile drug delivery solutions. Their comprehensive approach to intellectual property and innovation, backed by strategic partnerships and positive international assessments, highlights AGS as a company to watch. Through timely identification and monitoring of AGS’s advancements, KnowMade underscores the value of specialized patent monitoring services in providing actionable insights, enabling stakeholders to stay ahead in the evolving field of mRNA therapeutics and biopharmaceutical innovation.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
