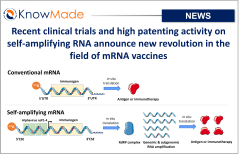
Recent clinical trials and high patenting activity on self-amplifying RNA announce new revolution in the field of mRNA vaccines
SOPHIA ANTIPOLIS, France – November 29, 2022 │ KnowMade has identified high recent IP activity in the field of self-amplifying RNA vaccines. This activity could be the sign of an upcoming revolution in the RNA vaccine market. Self-amplifying RNA is a next-generation platform for mRNA vaccines Compared with traditional vaccines (live-attenuated, inactivated, recombinant etc.), effective[…]