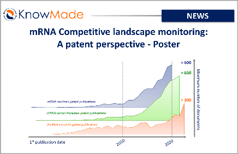
mRNA Competitive landscape monitoring: A patent perspective – Poster
SOPHIA ANTIPOLIS, France – November 15, 2023 │ KnowMade’s Therapeutic mRNA Patent Monitor was presented at the 11th mRNA health conference in Berlin (October 31 to November 2, 2023) through the poster N°49 (High resolution PDF download here). Abstract Therapeutic mRNA, which became widely known as vaccine against COVID-19, are expected to be applicated to[…]