
Bispecific Antibodies Mark a Breakthrough in Cancer Therapy Intellectual Property.
Publication January 2025
| Download Flyer | Download Sample |
Report’s Key Features
- IP trends, including time evolution of published patents, countries of patent filings and patents’ legal status
- Ranking of main patent assignees
- Key players’ IP position and relative strength of their patent portfolios
- Segmentation: Tumor Antigens (ERBB family, BCMA, BRCA, mesothelin, PSMA, CEA, claudin, EPCAM, mucin, NKG2D, VEGF, CEACAM, MAGE, ROR1, c-Met and nectin), Immune Checkpoints (PD1 / PDL1, CTLA-4, LAG-3, TIM-3, OX40, ICOS, B7-H3, TIGIT and BTLA) and T cells (CD3).
- Analysis of collaborations and EP patent oppositions.
- Excel database containing all patents analyzed in the report, including segmentations + hyperlink to updated online database (legal status, documents etc.)
Linked Reports
- Allogeneic CAR Patent Landscape Analysis 2023
- mRNA Cancer Therapies Patent Landscape 2022
- Self-amplifying RNA vaccines Patent landscape 2023
- Circulating DNA/RNA – Patent Landscape 2021
BsAbs offer exciting opportunities for the design and development of new drugs, and are expected to have lasting therapeutic impact
The development of bispecific antibodies (bsAbs) in oncology is experiencing rapid growth, accompanied by significant clinical advancements. According to recent data, more than 85% of bsAbs in clinical trials are cancer treatments, with approximately 600 bsAbs currently in clinical trials. To date, 11 bsAbs have received regulatory approval for use in cancer, ten of them by the US FDA. This expansion reflects the growing interest in these innovative therapeutic agents. Bispecific antibodies are engineered proteins designed to bind simultaneously to two distinct antigens. They can bridge two cell types (in-trans binding) or engage two molecules on the membrane of one cell (in-cis binding). BsAbs that bridge cells represent the largest group, with T cell redirection as the most common denominator. T-cell engagers bind both cytotoxic T cells and tumor cells, promoting a direct immune response against the tumor. Several bsAbs have recently received regulatory approvals. In December 2024, the U.S. FDA granted accelerated approval of a HER2 × HER3 bsAb for adults with advanced, unresectable, or metastatic non–small cell lung cancer (NSCLC) harboring a neuregulin 1 (NRG1) gene fusion with disease progression on or after prior systemic therapy, or advanced, unresectable, or metastatic pancreatic adenocarcinoma harboring an NRG1 gene fusion with disease progression on or after prior systemic therapy. However, challenges remain, such as the complexity of production, toxicities (cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, infusion-related reactions), and the need to optimize the stability and half-life of the molecules. Innovative approaches, such as advanced antibody engineering technologies and the development of new molecular formats, are being explored to overcome these obstacles. Understanding the intellectual property position and strategy of these various players is crucial in this evolving context. Detecting business risks and opportunities, anticipating emerging technologies, and enabling strategic decisions to strengthen market position can be achieved through this knowledge.
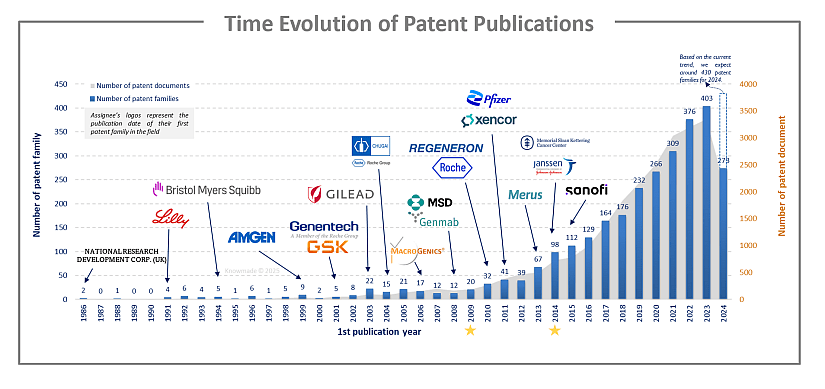
Between 1986 and the early 2000s, the number of patent publications is low but increasing. From 2010, the field is experiencing a significant acceleration, culminating in 2023 with more than 400 patent families. In the 1980s and 1990s and early 2000s, many advances were made in academic research such as the generation of the 1st asymmetric format, the 1st demonstration of T cell redirection, the 1st recombinant fragment-based formats, the 1st solution to light chain (LC) association issue through species-restricted LC pairing, the 1st solution to chain-association issue through use of complementary heavy chain (HC) (knobs into holes) and common LC, the 1st symmetric format, the discovery that natural human IgG4 is bispecific, the dual variable domain-Ig symmetric format pioneered, etc. All these innovations have contributed to the establishment of bsAbs. Then, in 2009, the bsAb catumaxomab (a T lymphocyte antigen CD3 × epithelial cell adhesion molecule (EpCAM)) received the European Union approval for the treatment of malignant ascites. Five years later, the blinatumomab (CD3×B lymphocyte antigen CD19) was FDA approved. It has been approved in the EU in 2015.
Analysis by segment
Bispecific Ab & Cancer have been investigated and the selected patent families labeled according to technologies to which they relate. This IP landscape features the following 3 types of segmentation: Tumor Antigens (ERBB family, BCMA, BRCA, mesothelin, PSMA, CEA, claudin, EPCAM, mucin, NKG2D, VEGF, CEACAM, MAGE, ROR1, c-Met and nectin), Immune Checkpoints (PD1 / PDL1, CTLA-4, LAG-3, TIM-3, OX40, ICOS, B7-H3, TIGIT and BTLA) and T cells (CD3).
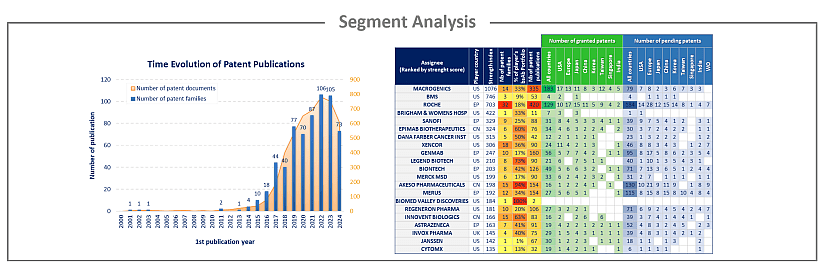
For each segment, the patent publication timeline, patenting strategy and patent portfolios of the main players have been analyzed.
EP oppositions
Currently, there is a significant number of EP oppositions which reflects the strategic issues of bispecific antibody & cancer for companies. For each opposed patent, the application date, assignee, opponent, opposition year, and results are detailed.
Identifying the companies that have recently emerged in the IP landscape
Among the players owning patent families related to Bispecific Ab & cancer, 52 newcomers were identified. These companies are either start-up firms (6) or established companies (46) developing their first technology in the field. Most IP newcomers are based in the U.S. and in Asia. It is possible that one of these innovative companies could become one of the next healthcare unicorns that the big corporations will be tempted to acquire.
IP profile of key players
This IP study includes a selection and description of main players. The patent portfolio analysis of main players includes a description of the assignee, patent portfolio description, time evolution of patent publication, main geographical coverage and live patents by technical segment. This IP profile overview is followed by the description of the technological content of their key patents and by a table with its clinical trials.
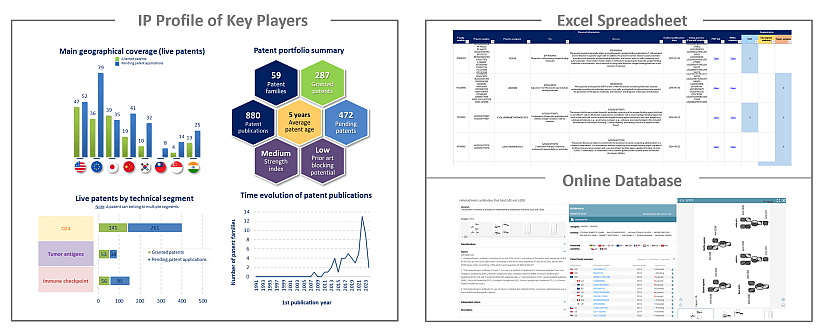
Moreover, the report includes an Excel spreadsheet with the 2895 patent families analyzed in this study. This useful patent database allows for multi-criteria searches and includes patent publication numbers, hyperlinks to the original documents, priority dates, titles, abstracts, patent assignees, each patent’s current legal status and segmentation. The report also includes a Patent Online Database which legal status are updated for each patent document.
Companies mentioned in this report (non-exhaustive list)
ROCHE, AMGEN, JANSSEN, GENMAB, XENCOR, REGENERON PHARMACEUTICALS, GENENTECH – ROCHE, MACROGENICS, DRAGONFLY THERAPEUTICS, SANOFI, CHUGAI PHARMACEUTICAL, MERCK MSD, MERUS, BMS, SAMSUNG, JIANGSU HENGRUI PHARMACEUTICALS, ABBVIE, HEFEI TG IMMUNOPHARMA, MARENGO THERAPEUTICS, etc.
