SOPHIA ANTIPOLIS, France – February 04, 2025 │ KnowMade actively tracks therapeutic mRNA innovations as part of its Therapeutic mRNA patent monitoring services. What’s new today?
CHMP Recommends Approval of KOSTAIVE® (ARCT-154)
EMA opinion
The European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion recommending the approval of KOSTAIVE®, a self-amplifying mRNA (sa-mRNA) vaccine developed collaboratively by Arcturus Therapeutics, CSL, and Meiji Seika Pharma. Designed for the prevention of COVID-19 in adults aged 18 and older, this vaccine represents a significant advancement in mRNA technology, previously identified in KnowMade’s analyses as a key innovation in vaccines development (see the Self-Amplifying RNA vaccine patent landscape from 2023). KOSTAIVE® utilizes sa-mRNA technology, which enhances immune responses by enabling cells to amplify mRNA and protein production. This advancement offers key benefits, including lower dosage requirements, potentially fewer side effects, and durable immunity. According to CHMP’s evaluation, the vaccine demonstrated significant efficacy and safety in clinical studies. Results showed a reduction in symptomatic COVID-19 cases after two doses compared to placebo, alongside strong neutralizing antibody responses and minimal side effects as illustrated in figure 1 (EMA evaluation and Nhân Thị Hồ et al., 2024).
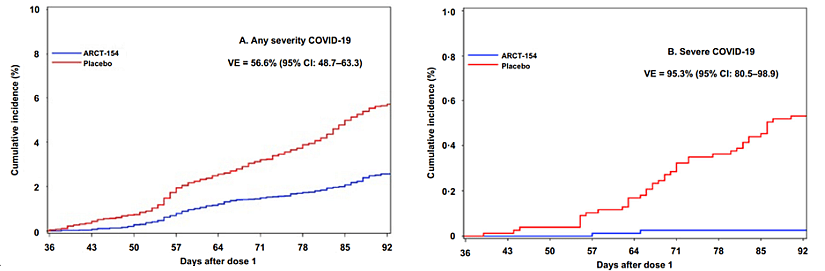
Figure 1: Cumulative incidence curves of COVID-19 of any severity (A), and severe COVID-19 (B) in vaccine and placebo groups from Day 36. From Nhân Thị Hồ et al., 2024.
The development of KOSTAIVE® is the result of a global collaboration. Arcturus Therapeutics, a leader in mRNA-based medicines for infectious diseases and rare conditions, partnered with CSL and Meiji Seika Pharma to advance this vaccine from clinical trials to commercialization. Arcturus has also been actively involved in advancing mRNA technology through other vaccine candidates and therapeutic pipelines. Notably, Arcturus develops its own mRNA vaccine platform already detailed in KnowMade’s insight here.
Clinical Trials and Safety Validation
The CHMP’s positive opinion follows extensive clinical trials that confirmed the vaccine’s safety, tolerability, and robust immune response. Preclinical research demonstrated that ARCT-154 elicited strong neutralizing antibody responses against multiple COVID-19 variants, including Alpha, Beta, Delta, and Gamma, in non-human primates. Clinical trials were conducted in multiple countries. In Singapore, a Phase I-II clinical trial, partially funded by the Singapore government, was approved in August 2021 to evaluate ARCT-154 and ARCT-165 as both a primary vaccination and a booster following Pfizer-BioNTech vaccination. Meanwhile, in Vietnam, Vinbiocare, a subsidiary of Vingroup, fully sponsored the clinical trials, which involved over 21,000 participants across three phases. Phase I, launched in August 2021 at Hanoi Medical University, assessed safety in 100 volunteers, while Phases II and III were conducted across Bắc Ninh, Hanoi, and Long An, involving over 1,000 volunteers. In Japan, a Phase III non-inferiority trial randomized 828 participants to receive either ARCT-154 or the Pfizer-BioNTech vaccine, with results published in December 2023 (Yoshiaki Oda et al., 2023). Additionally, a Phase III COVID-19 booster trial confirmed that ARCT-154 achieved higher immunogenicity compared to a standard mRNA vaccine, reinforcing its potential as a next-generation COVID-19 vaccine. These comprehensive clinical studies validated the vaccine’s strong safety profile, high immunogenicity, and effectiveness, contributing to its approval in Japan and positioning it as a key player in global COVID-19 vaccination efforts.
Arcturus Technology
Self-Amplifying platform
The IP of Arcturus therapeutic protecting the sa-mRNA platform was describe in KnowMade insight from January 2023 (here), focused on delivery. Briefly, Arcturus’s sa-mRNA vaccine platform allows smaller doses to achieve robust immune responses by using saRNA, which replicates intracellularly to produce antigens over an extended period. Key technical innovations are detailed in Arcturus’ patent families. For example, WO2020191103 describes methods for producing LNPs encapsulating long mRNAs (800–12,000 nucleotides) with high encapsulation efficiency (95–97%) using optimized lipid formulations. Similarly, WO2022056413 improves upon this by addressing the challenges of encapsulating larger mRNAs, employing precise flow rates to reduce shear forces and preserve RNA integrity. Lyophilization methods, outlined in WO2022036170, ensure the stability of LNP formulations during long-term storage, achieving 93% sa-mRNA encapsulation efficiency even after reconstitution. To date, these patent families include granted U.S. patents and pending applications with broad international coverage, as outlined in the following table.
| PATENT FAMILY | 1ST APPLICATION DATE | GRANTED MEMBERS | PENDING MEMBERS |
| WO2020191103 | 2020-03-18 | US11737979 | EP, CA, BR, SG, IL, AU, CN, VN, KR, JP, IN |
| WO2022056413 | 2021-09-13 | US11938227 | EP, CA, BR, SG, IL, AU, MX, CN, VN, KR, JP, ID, IN |
| WO2022036170 | 2021-08-13 | US12178921 | EP, AU, BR, CA, CN, ID, IL, IN, JP, KR, MX, SG, VN |
In addition to the key patent families protecting its self-amplifying mRNA (sa-mRNA) platform, Arcturus Therapeutics holds patent families covering the sa-mRNA vaccine itself and published since 2021.
The patent family WO2021/183563, titled “Coronavirus Vaccine Compositions and Methods”, published on March 9, 2021 describes nucleic acid molecules encoding both viral replication proteins and antigenic coronavirus proteins or their fragments. The first independent claim as filed is “1. A nucleic acid molecule comprising: (i) a first polynucleotide encoding one or more viral replication proteins, wherein the first polynucleotide is codon-optimized as compared to a wild-type polynucleotide encoding the one or more viral replication proteins; and (ii) a second polynucleotide comprising a first transgene encoding a first antigenic protein or a fragment thereof, wherein the first antigenic protein is a coronavirus protein.”. Currently, this patent family is granted in the U.S. and pending in multiple jurisdictions (AU, CA, EP, HK, JP, and TW).
In addition, the patent family WO2023/010128, titled “RNA Vaccines”, published on July 29, 2022 describes RNA molecules encoding both viral replication proteins and antigenic proteins or fragments thereof. The first independent claim as filed is “An RNA molecule comprising: (a) a first polynucleotide encoding one or more viral replication proteins, wherein one or more miRNA binding sites in the first polynucleotide have been modified as compared to a reference polynucleotide; and (b) a second polynucleotide comprising a first transgene encoding a first antigenic protein or a fragment thereof.”. Unlike the previous patent family, WO2023/010128 remains pending in all jurisdictions with an extensive international coverage (AR, AU, BR, CA, CN, CR, EAPO EA, EP, HK, ID, IL, IN, JP, KR, MX, NZ, PH, SG, TW, and US).
Coronavirus vaccines
The current pipeline of Arcturus comprises three COVID-19 vaccines and two influenza vaccines. Four of them are developed in collaboration with CSL, an Australian multinational specialty biotechnology company (see figure 2). The collaboration and licensing agreement was closed in December 2022 (Press release here). This collaboration firstly results in Japanese approval of ARCT-154, the first Self-Amplifying mRNA vaccine approved for COVID in adults, in November 2023, marking a historic milestone for CSL and Arcturus Therapeutics. Based on robust clinical data demonstrating higher immunogenicity and safety compared to standard mRNA vaccines, this approval is the first major achievement since their 2022 collaboration agreement. Distributed in Japan by Meiji Seika Pharma, ARCT-154 strengthens CSL’s innovative vaccines portfolio and underscores the potential of sa-mRNA technology in combating COVID-19 and other infectious diseases.
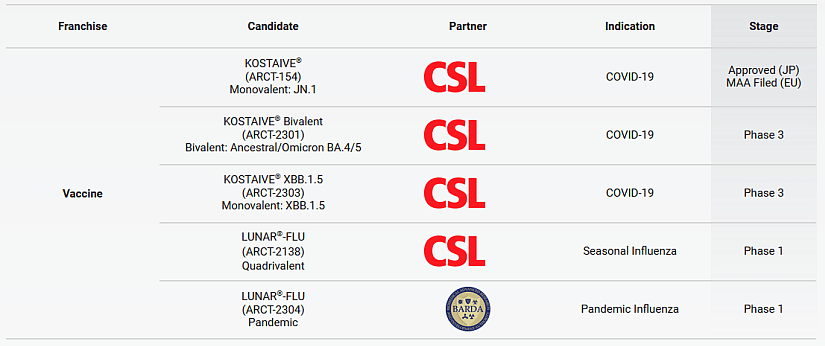
Figure 2: Pipeline of Partnered mRNA Therapeutics and Vaccines
Arcturus has partnered with several industry leaders and has a robust and diverse preclinical drug development pipeline. In collaboration with Arcturus development partners, the company is leveraging the LUNAR® platform to develop RNA medicines for diseases with significant unmet medical needs and accelerated clinical paths. From Arcturus web site.
Why subscribe to this monitor?
A patent monitoring service is an essential asset for businesses striving to stay ahead in innovative fields such as mRNA therapeutics. Our tailored solutions provide timely and curated patent intelligence, equipping organizations with the tools to track technological breakthroughs, identify collaboration opportunities, and protect their innovations from infringement risks. By leveraging our bespoke IP monitoring services, companies gain actionable insights that support informed decision-making, enabling them to navigate the complexities of the intellectual property landscape with confidence. This strategic approach fosters growth and drives innovation across industries.
As a leader in patent analysis and monitoring, KnowMade closely follows such milestones, providing insights and solutions to support stakeholders in the dynamic mRNA vaccine landscape. For more detailed insights into these developments and how they may impact your business, please contact us.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
