SOPHIA ANTIPOLIS, France – September 03, 2024 │ The last quarterly report for the 2024 Therapeutic mRNA patent monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics.
Monkeypox virus, a new threat?
Mpox, also known as monkeypox, is a viral disease caused by the monkeypox virus, part of the Orthopoxvirus genus. It can lead to symptoms such as fever, rash, and swollen lymph nodes, and in severe cases, it can be fatal. The disease spreads through close contact with infected individuals, contaminated materials, or animals. Recent outbreaks, particularly in Africa and globally, have raised significant health concerns due to the virus’ potential for severe illness and rapid transmission, leading to international public health emergencies. According to WHO, over 120 countries have reported mpox between Jan 2022 – Aug 2024, with over 100 000 laboratory-confirmed cases reported and over 220 deaths among confirmed cases (see WHO web site here).
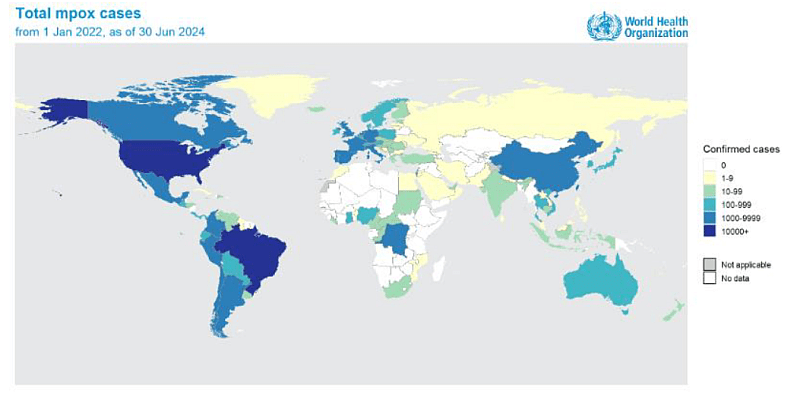
Figure 1: Geographic distribution of confirmed cases of mpox reported to or identified by WHO from official public sources, from 1 January 2022 to 30 June 2024. From WHO.
Currently, there is no cure for Mpox, but ongoing efforts conducted by scientists and global health organizations are focused on developing treatments and diagnostics. Two vaccines are available for emergency use in the Democratic Republic of Congo (DRC), produced by Denmark’s Bavarian Nordic and Japan’s KM Biologics, aimed at reducing infection risk. In addition, various next-generation mRNA-based vaccines are in development. Among major players within the mRNA vaccination field, BioNtech and Moderna are the one with IP rights related to Mpox mRNA vaccine.
BioNTech in partnership with CEPI to develop mRNA Mpox vaccine
BioNTech, has partnered with the Coalition for Epidemic Preparedness Innovations (CEPI), an organization dedicated to accelerating the development of vaccines for emerging infectious diseases (see press release here). This partnership centers on advancing BioNTech’s BNT166 program, which aims to develop an mRNA-based vaccine for Mpox (formerly monkeypox). CEPI is committed to providing up to $90 million in funding to support the development of this vaccine, aligning with its 100 Days Mission (i.e., a global goal to accelerate development of well-tolerated and effective vaccines against a potential future pandemic virus so that a vaccine can be ready for regulatory authorization and manufacturing at scale within 100 days of recognition of a pandemic pathogen).
The collaboration between BioNTech and CEPI not only focuses on the immediate goal of creating a safe and effective Mpox vaccine but also aims at contributing broadly to the global vaccine portfolio against Orthopoxvirus family. The data and insights gained from this program will help to respond rapidly to future pandemics. Moreover, this partnership reflects BioNTech’s commitment to equitable access to vaccines, particularly in low- and middle-income countries. The strategic alignment with CEPI’s mission underscores the potential impact of this collaboration on global health security, potentially preventing future outbreaks and enhancing the world’s preparedness against pandemics. Recently, BioNTech and CEPI expanded their partnership to strengthen Africa’s mRNA vaccine ecosystem by supporting the expansion of a facility in Kigali, Rwanda.
The BNT166 vaccine
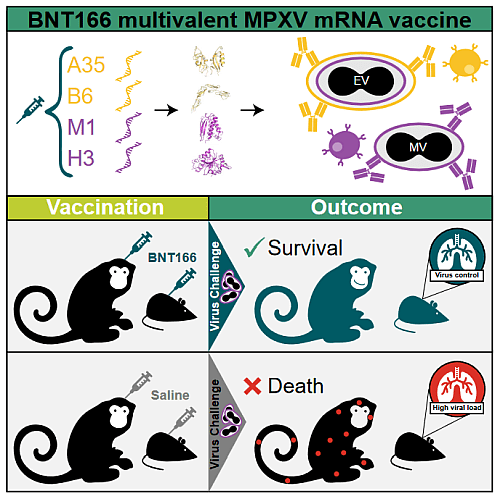
Figure 2: Graphical abstract of BNT166 multivalent MPXV mRNA vaccine test From Zuiani et al., 2024
BioNTech already started a Phase 1/2 clinical trial of the BNT166 on 2023-09-21. The clinical trial NCT05988203 trial compares two candidates, BNT166a and BNT166c, to determine which one is safer, more tolerable, and more effective in generating immunity. The BNT166a and BNT166c vaccine candidates differ in the specific viral antigens they target on the Mpox virus, but both encode surface protein of two different form of the virus. In February 2024, scientific results detailing BNT166a and BNT166c efficiency was released (Zuiani et al., 2024) and are summarized in the figure 2. This work details the structure of both candidates and their efficiency in two animals’ models, mice and macaques. Both BNT candidates (BNT166a and BNT166c) are multivalent vaccines encoding antigens from both extracellular virions (A35 and B6) and mature virions (M1 and H3), but BNT166a is a quadrivalent vaccine (encoding the Mpox antigens A35, B6, M1, H3) while BNT166c is a trivalent vaccine (without H3). In mice, both candidates induced robust T cell responses and IgG antibodies, including neutralizing antibodies to both Mpox and vaccinia virus. In a model that better represents human mpox, including development of lesions, authors used a non-human primate (NHP) Mpox challenge model. The vaccinated group received two doses of BNT166a, which generated high IgG titers against mpox antigens and serum-neutralizing antibodies. Following a lethal mpox challenge, all vaccinated animals survived with mild symptoms and minimal lesion development, while 83.3% of the unvaccinated group succumbed to the infection. These results demonstrate that BNT166a protects mice and macaques from Monkeypox and suggest a broader protection from Orthopoxvirus.
Regarding the IP rights, BioNTech filed an international patent application on 2023-05-25 related to the BNT166 vaccine, WO2023/230295. The patent application provides monkeypox antigen constructs that can be used in a Mpox vaccine, pharmaceutical compositions comprising mRNA(s) encapsulated within a LNP and related technologies. Despite numerous antigens are disclosed in the patent application, the experimental part only focused on five combinations of antigens, combo 1 to 5. Combo 4 corresponds to BNT166a while combo 2 corresponds to BNT166c (see table below). Experimental part of the patent application disclosed data confirming a broad Orthopoxyirus protection. Indeed, BALB/c mice were immunized with the five compositions (combo1 to 5) prior to challenge with vaccinia virus that belongs to the same family, Poxviridae, of Monkey Pox virus. After 8 days post vaccinia challenge, mice immunized with saline control showed 100% mortality while mice immunized with combo 1 to 5 show 100% survival, as illustrated in figure 3.
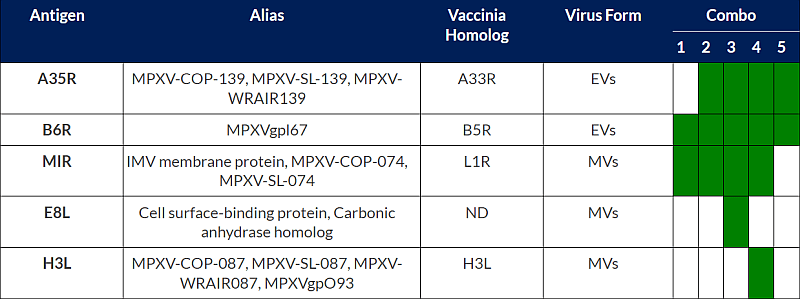
Table1: Mpox antigens encoded by multivalent vaccines tested in WO2023/230295.
In addition to Mpox antigens used in combo 1 to 5, the table gives antigens from combo 1 to 5 and their alias used in bibliography, the name of their homolog in another model of Poxviridae, the vaccinia virus, and also the specificity of antigens extracellular virions (EVs) or mature virions (MVs).
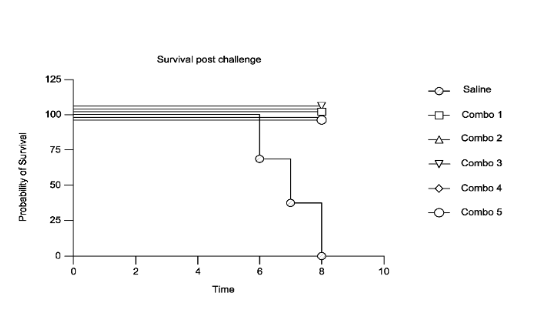
Figure 3: Survival curve
The figure shows percent survival of BALB/c mice immunized with compositions comprising constructs encoding combinations of antigens (Combo 1, Combo 2, Combo 3, Combo 4, Combo 5) or saline before challenge with vaccinia virus. Combo 1 comprises B6 and Ml antigens; Combo 2 comprises A35, B6, and Ml antigens; Combo 3 comprises A35, B6, Ml, and E8 antigens; Combo 4 comprises A35, B6, Ml, and H3 antigens; and Combo 5 comprises A35 and B6 antigens. From WO2023/230295.
The first claim of this patent application is as follows:
1. A composition comprising a polyribonucleotide encoding one or more monkeypox antigens or fragments thereof and a pharmaceutically acceptable carrier.
The ISA noted that claim 1, which pertains to a composition comprising a polyribonucleotide encoding one or more monkeypox antigens, does not meet the requirements of Article 33(3) of the PCT, as it lacks inventive step. In particular, the ISA emphasized that the use of an mRNA-based composition for a monkeypox vaccine would be obvious to a person skilled in the art, especially in light of prior art documents such as D1 (Heraud et al., 2006) and D2 (Corbet al., 2020). The ISA also mentioned that the examples in the application only demonstrate effectiveness for specific combinations of polyribonucleotides, and there is no support for such a broad claim.
Therefore, the first claim of the patent application will likely need to be amended to be limited to specific compositions demonstrated as effective, including requirements that the polyribonucleotides be formulated in LNP and restricting the specific sequences of polyribonucleotides to those described in the examples.
BioNTech & Moderna, still in competition
Moderna is also developing a mRNA vaccine to prevent Mpox infection, the mRNA-1769 which is still in phase 1/2 (NCT05995275, started in August 2023). Interestingly, mRNA-1769 is also a tetravalent vaccine encoding antigens A35r, B6R and M1R. Nevertheless, it also comprises an mRNA encoding another antigen, the surface protein of mature virion A29L (ortholog of A27L in vaccinia virus). An international patent application covering this vaccine, WO2023/230481, was filed two days prior to the BioNTech international patent application, on the 2023-05-23.
The first claim of this patent application is as follows:
1. A composition, comprising: a first messenger ribonucleic acid (mRNA) polynucleotide comprising an open reading frame (ORF) encoding a first orthopoxvirus protein and a lipid nanoparticle.
The first claim of this patent application, which pertains to a composition comprising an mRNA encoding for an orthopoxvirus protein and a lipid nanoparticle, will likely need to be modified to specify the specific orthopoxvirus proteins (such as A27L, M1R, B6R, and A35R) and to specify the composition of the lipid nanoparticles. These modifications would restrict the scope of the claim to specific, potentially novel and non-obvious combinations, in line with the description of the patent, in order to better meet the requirements of novelty and inventive step.
The patent applications filed by BioNTech and Moderna demonstrate similar (mRNA vaccines with LNPs) but distinct approaches in the combinations of antigens used, which could influence the effectiveness of protection of these vaccines against Mpox infection. Furthermore, the close filing dates of these two patent applications highlight that BioNtech and Moderna continue to compete in the rapidly developing field of mRNA therapies, particularly in regards to vaccines for emerging infectious diseases. Both companies are considered as main players in an IP point of view, see our last insight on the 2023’s patenting activity.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
Brice Sagot, CEO and co-founder of KnowMade, leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
