SOPHIA ANTIPOLIS, France – November 29, 2022 │ KnowMade has identified high recent IP activity in the field of self-amplifying RNA vaccines. This activity could be the sign of an upcoming revolution in the RNA vaccine market.
Self-amplifying RNA is a next-generation platform for mRNA vaccines
Compared with traditional vaccines (live-attenuated, inactivated, recombinant etc.), effective mRNA-based vaccines can be rapidly designed and manufactured at an industrial scale while remaining low cost. With these advantages, mRNA vaccines have taken the lead in the race for a COVID-19 vaccine, and have proven their efficiency over conventional vaccines for this condition. Moreover, mRNA vaccines are already being investigated in clinical trials for other infectious diseases and different cancers. However, the decrease in immune response to these mRNA vaccines and the continued emergence of COVID-19 variants now necessitate the use of regular booster doses. There is therefore a need to develop more potent mRNA vaccines while maintaining a significant safety level. Currently, one of the most promising technologies to develop next-generation mRNA vaccines is the use of self-amplifying RNA (saRNA), also referred to as self-replicating RNA (srRNA). As depicted in the diagram below, the backbone of saRNA, typically derived from an alphavirus genome, encodes a gene of interest and a viral replicase (RNA-dependent RNA polymerase), which can amplify the genomic and subgenomic RNA.
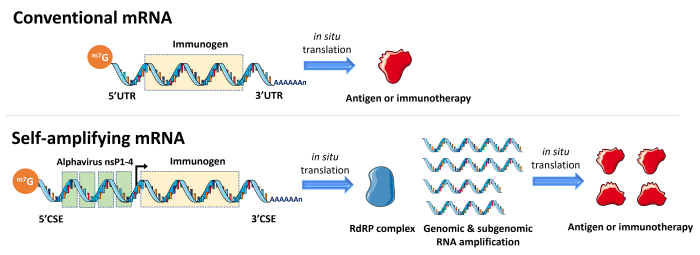
Conventional and self-amplifying RNA vaccine designs. The saRNA vaccine contains additional alphavirus replicon genes and conserved sequence elements. The nonstructural proteins 1, 2, 3 and 4 (nsP1-4) are essential for replicon activity as they form the RdRP complex (RNA-dependent RNA polymerase or RNA replicase). Adapted from Bloom et al., 2021.
saRNA advantage over traditional mRNA
The self-amplification properties enable use of a much lower dose of saRNA compared to mRNA, typically 100-fold lower. In addition, duration of expression from saRNA is longer than that obtained with mRNA, with the ability to last almost two months in vivo when expressing a reporter gene (Geall et al., 2012). Furthermore, saRNA vaccines mimic a viral infection through their replicase activity leading to dsRNA. This elicits a broader immune response, which can be considered to have potential to be effective with a single-dose inoculation. Moreover, like conventional mRNA, saRNA is a versatile platform, as it is possible to develop other therapeutic applications – including infectious diseases such as influenza, rabies, HIV-1, malaria, Chlamydia trachomatis, Ebola, RSV and Zika viruses – as well as oncology applications, such as melanoma and prostate cancer.
Clinical trials on saRNA vaccines against COVID-19
In terms of pipeline development, several clinical trials investigating the safety and efficacy of saRNA vaccines against COVID-19 are currently ongoing (Imperial College London, Elixirgen Therapeutics, Arcturus Therapeutics, GSK, VLP Therapeutics, Gritstone bio, etc.). The first-in-human clinical trial for an saRNA vaccine against COVID-19 was carried out in 2020 by Imperial College London. The doses used in the trial, ranging from 0.1 to 10 µg, compared to 30 µg for the Pfizer/BioNTech vaccine and 100 µg for the Moderna vaccine, illustrate the efficiency of this platform. These doses would enable 10–1,000 times as many doses from the same batch of RNA. More recently, in October 2022, Gritstone bio announced positive Phase 1 results from its ongoing trials evaluating its self-amplifying mRNA vaccine candidates against SARS-CoV-2. Thus, although saRNA technology has long been known through alphavirus replicons, mRNA vaccines against COVID-19 have shed particularly exciting new light on saRNA-based vaccines, and there has been a lot of recent academic and industrial work on the topic.
Recent patenting activity on saRNA is high
Regarding patent applications, replicons and saRNA-based vaccines have received highly significant renewed interest since the COVID-19 pandemic and the advent of RNA vaccines. About 400 patent families describing saRNA-related technologies have been published since the 90s. As depicted in the graph below, the number of patent applications published in 2021-2022 tripled in comparison to previous years.
Among these patent applications published in 2021 and 2022, many focus on vaccination against COVID-19. However, other applications are also claimed, such as vaccines against other infectious diseases (such as influenza, herpes, HIV etc.), prophylactic/therapeutic treatments against various cancers (e.g., prostate cancer or melanoma), or vaccines for veterinary applications, mainly filed by Intervet (MSD Animal Health). Furthermore, challenges arise in trying to apply conventional methods to form lipid-encapsulated RNA nanoparticles from large RNAs, such as saRNA. For example, large mRNAs might be about 6,000 to 15,000 nucleotides in size. These large nucleotides may not be compatible with typical LNP forming processes. As such, some patent applications focus on this new technical issue, such as patent publication WO2022056413, filed by Arcturus Therapeutics.
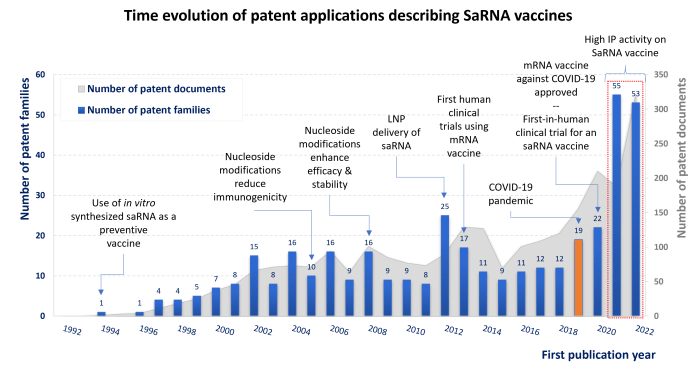
Time evolution of patent applications describing saRNA vaccines and key milestones related to both general advancement of mRNA technology and the evolution of saRNA as a vaccine platform.
Moderna, BioNTech and CureVac are not well positioned on this technology
It is also interesting to observe that the main players leading conventional RNA vaccines (i.e., Moderna, BioNTech and CureVac) are weak or not present in the field of saRNA, which is instead led by players such as GSK, Gritstone or Janssen Biotech. It is also interesting to observe the arrival of several startups focusing on this technology, such as VaxEquity, a UK biotechnology company developing self-amplifying RNA therapeutics and vaccines, or Replicate Bioscience, a US company which has raised $40 million in Series A funding, creating novel oncology treatments to prevent and reverse drug resistance through its saRNA platform.
saRNA represents a new revolution in the field of mRNA vaccines
If the results of clinical trials for these different applications overcome those obtained with conventional mRNAs, the insignificant presence of CureVac, Moderna or BioNTech from an IP point of view will significantly redraw the mRNA technology landscape. In the advent of RNA vaccines from Pfizer-BioNTech and Moderna supplanting traditional vaccines during the COVID-19 pandemic, we may be witnessing a new revolution in the field of RNA vaccines. The details of these players, their IP strategies and their patented technologies will be analyzed in a forthcoming report to be published by KnowMade in 2023.
All patent landscape reports revolving around healthcare.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Brice Sagot, CTO and co-founder of Knowmade, leads the Biotechnology and Life Sciences department. He holds a PhD in Molecular Biology from the University of Nice SophiaAntipolis (France).
About Knowmade
Knowmade is a Technology Intelligence and IP Strategy consulting company specialized in analysis of patents and scientific information. The company helps innovative companies and R&D organizations to understand their competitive landscape, follow technology trends, and find out opportunities and threats in terms of technology and patents.
Knowmade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patents and scientific information into business-oriented report for decision makers working in R&D, Innovation Strategy, Intellectual Property, and Marketing. Our experts provide prior art search, patent landscape analysis, scientific literature analysis, patent valuation, IP due diligence and freedom-to-operate analysis. In parallel the company proposes litigation/licensing support, technology scouting and IP/technology watch service.
Knowmade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Solid-State Sensors/Actuators, Semiconductor Manufacturing, Packaging & Assembly, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
