SOPHIA ANTIPOLIS, France – March 7, 2025 │ A few days ago, Regeneron decided to no longer seek accelerated approval of odronextamab, a bispecific antibody (bsAb) CD20 x CD3, for relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). However, the company is continuing to pursue this approval for follicular lymphoma (FL), with an FDA decision expected by July 30, 2025. Regeneron is justifying its withdrawal of accelerated approval for DLBCL by “competitive developments.” The company now plans to seek full approval for odronextamab in DLBCL, but only after the results of ongoing Phase III studies are published.
The company
Regeneron is founded in 1988 by Leonard S. Schleifer, a young neurologist and assistant professor at Cornell University Medical College. Regeneron’s medicines and pipeline are designed to treat patients with eye diseases, allergic and inflammatory diseases, cancer, cardiovascular and metabolic diseases, neurological diseases, hematologic conditions, infectious diseases and rare diseases. It accelerates drug development using its proprietary technologies, such as VelociSuite®. Regeneron uses its platforms to design minimally engineered, fully human bsAb medicines that resemble naturally occurring antibodies as closely as possible. Veloci-Bi® allows for the generation of full-length bsAb medicines that can be made via standard manufacturing techniques with the goal of having favorable properties similar to natural antibodies. In August 2024, European Commission has approved Ordspono™ (odronextamab) to treat adult patients with R/R follicular lymphoma or R/R diffuse large B-cell lymphoma.
To protect its innovative technologies, Regeneron has built a significant patent portfolio covering its developments in the field of bsAbs, particularly in oncology, where its therapies aim to harness the immune system to fight cancer.
Regeneron’s patent portfolio
Regeneron has 49 patent families identified in bispecific antibody for oncology treatment (see KnowMade’s report: Bispecific Antibody & Cancer Patent Landscape Analysis), published between 2010 and 2024. This patent portfolio (figure 1) represents more than 800 documents with worldwide applications (Europe, USA, Asia). 89 % are alive patent families (pending applications and granted patents). For now, there is no EP opposition or US litigation identified.
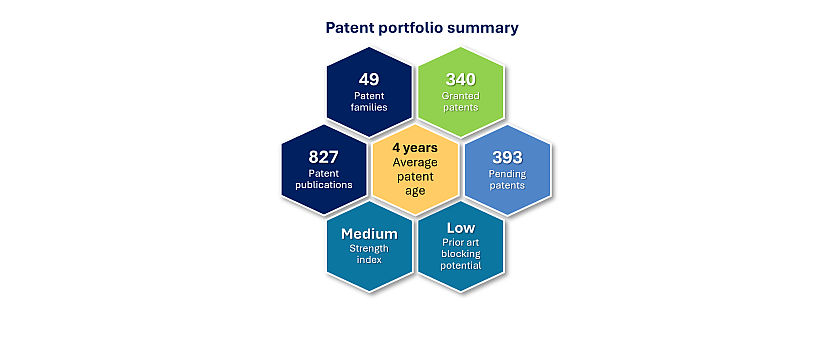
Figure 1: Regeneron’s patent portfolio in bsAbs for cancer treatment.
The company is mainly focused on bsAb with an “obligate” mechanism which is the redirection of immune cells to cancer cells to eliminate them. Indeed, it developed bsAb targeting a tumor antigen (TA) in combination with a T cell (CD3 or CD28). By this approach, T cells are physically linked with tumor cells via bsAbs (figure 2). The main bsAbs identified in Regeneron’s patent portfolio are: CD20 x CD3, BSMA x CD3, Muc16 x CD3 or CD28, PSMA x CD3 or CD28 and EGFR x CD28.
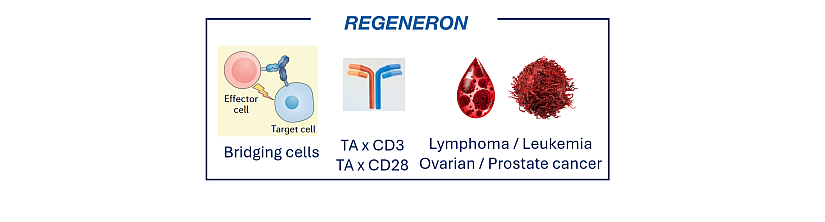
Figure 2: Overview of Regeneron’s patented technologies in bsAbs for cancer treatment.
Odronextamab
Odronextamab (REGN1979) is a hinge-stabilized, human CD20 x CD3 IgG4-based bispecific antibody that binds CD20-expressing cells and CD3 on T cells, targeting CD20+ cells via T-cell-mediated cytotoxicity, independent of T-cell receptor-mediated recognition (figure 3).
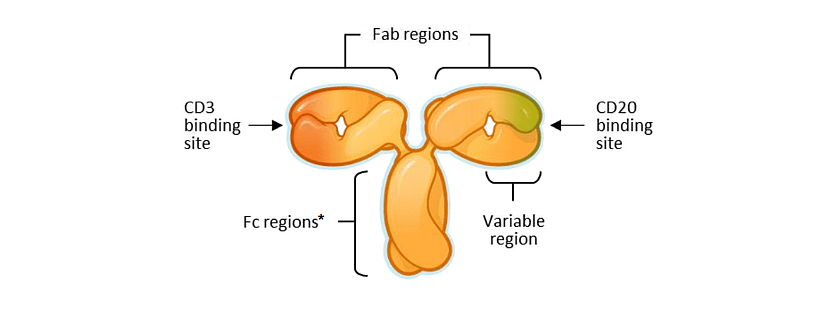
Figure 3: Schematic representation of the bsAb odronextamab from Regeneron.
Cytokine Release Syndrome (CRS)
Regeneron owns 8 patent families describing CD20 x CD3 bsAbs. The three last patent families, published between 2019 and 2023, are alive and describe a method of treatment against cancer and to reduce cytokine release syndrome. CRS is a systemic inflammatory response that can be triggered by certain drugs. T cell-activating cancer immunotherapies carry a particularly high risk of CRS, which is usually due to on-target effects induced by binding of a bsAb or chimeric antigen receptor (CAR) T cell to its antigen and subsequent activation of bystander immune cells and non-immune cells, such as endothelial cells. Activation of the bystander cells results in the massive release of a range of cytokines. IL-6, IL-10, and interferon (IFN)-y are among the core cytokines that are consistently found to be elevated in the serum of patients with CRS. With T cell-activating therapies directed against tumor cells, CRS is triggered by the massive release of IFN-y by activated T cells, or the tumor cells themselves. Secreted IFN-y induces activation of other immune cells, most importantly macrophages, which in turn produce excessive amounts of IL-6, TNF-α, and IL-10. In some cases, the symptoms associated with CRS are termed infusion-related reaction (IRR) if they occur less than 6h following the start of infusion, and CRS if they occur from 6h onward following the start of infusion. Signs and symptoms of CRS include fever, nausea, headache, rash, rapid heartbeat, low blood pressure, and trouble breathing. Most patients have a mild reaction, but sometimes, the reaction may be severe or life threatening. The management of the toxicities of cancer immunotherapy is a challenging clinical problem.
How does Regeneron prevent severe CRS with odronextamab?
The patent family published in 2019 has granted patents in Europe, the USA, Japan, etc. and pending applications in Canada, Australia, China, Korea, etc. The company provides a dosing strategy to mitigate CRS (EP3844189) in patients with CD20+ DLBCL, refractory to CAR-T therapy or that have relapsed following prior CAR-T therapy. The methods employ initial fractional dosing with optional administration of additional agents such as steroids or cytokine antagonists that are discontinued with maximal weekly dosing over the course of the dosing regimen. The cumulative odronextamab safety and pharmacokinetics experience through the dose-limiting toxicities evaluation period of 27 mg REGN1979 demonstrates that the management algorithm for CRS or IRR reactions (i.e., incremental dose escalation, split dosing during the initial weeks of REGN1979 administration, and premedication with corticosteroid) has proved effective in preventing severe CRS or IRR despite incremental increases in dosing in successive dose cohorts. Split dosing provided a benefit to patient safety in weeks 1 through 4 (the available data), wherein less overall incidents of severe CRS/IRR were observed. Particularly, the dosing strategy discussed herein provided a safer strategy for escalating doses to levels greater than 80 mg, even 160 mg or greater, with less severe events occurring in weeks 3 and 4 when higher doses reached and exceeded the desired serum concentrations discussed above.
In 2021, Regeneron filed a patent application (pending patent applications in Europe, the USA and Asia) disclosing a method to administering anti-CD20/anti-CD3 bsAb before administering to the cancer subject cemiplimab, an Ab that specifically binds programmed death 1 (PD-1) (WO2022/072762). This method inhibits tumor growth and ameliorates CRS in the subject. This disclosure is based, at least in part, on the unexpected discovery that pretreatment of the T-cells with odronextamab and delaying exposure to an anti-PD-1 Ab (cemiplimab) led to a marked decrease in cytokine (e.g., IL-2) release upon exposure to the combination of odronextamab plus cemiplimab, compared to control T-cells that had not undergone odronextamab pretreatment. This reduction in cytokine levels seen after odronextamab pretreatment supports the conclusion that initiating odronextamab before adding cemiplimab can help to significantly mitigate CRS.
Then, in 2023, the company disclosed a dosing regimen for administering odronextamab to treat a B-cell malignancy in patients (pending patent applications in Europe, the USA and Asia – WO2023/164143). The methods employ fractional dosing over several weeks of treatment along with administration of a steroid and an antihistamine. In an example, an intravenous (IV) odronextamab step-up regimen for reducing the risk of high-grade CRS is performed. The step-up dosing regimen consists of 0.7 mg (0.2/0.5 mg split over 2 days) at Week 1, 4 mg (2/2 split) at Week 2, and 20 mg (10/10 split) at Week 3. Early administration of premedication (12-24 hours prior to first split dose and on the day of each split dose vs. only on the day of each split dose) significantly reduced baseline (pre-treatment) cytokine levels. This new regimen has been tested in clinical studies in patients with follicular lymphoma and diffuse large B-cell lymphoma.
As of April 2022, there have been no Gr≥3 CRS in the first 100 patients treated with this regimen. Importantly, although the odronextamab IV step-up dosing regimen showed a lower concentration of odronextamab compared with the original regimen during Weeks 1-3, the concentrations were similar after the first full dose was administered, with similar trough concentrations after Week 4. This indicates that the same therapeutic levels are achieved with both regimens, which is beneficial for the treatment of disease.
To conclude, Regeneron continues to strengthen its position in the field of bsAbs, notably with odronextamab, despite the withdrawal of its accelerated approval application for DLBCL due to competition. The company is now focused on full approval following completion of Phase III studies, while continuing the approval process for follicular lymphoma, with an FDA decision expected by July 2025. Its patent portfolio demonstrates its commitment to developing innovative strategies to maximize the efficacy of bsAbs while limiting side effects, in particular cytokine release syndrome. Through optimized dosing protocols and combination approaches with other antibodies, Regeneron improves the safety and efficacy of its therapies. This strategic approach, combined with strong intellectual property protection, gives the company a key competitive advantage in the field of oncology.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for Knowmade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
