SOPHIA ANTIPOLIS, France – April 11th, 2025 │ On March 19th, 2025, a collaboration was announced between Oxford BioTherapeutics (OBT) and Roche to discover novel potentially first-in-class antibody-based therapeutics for the treatment of cancer. Targets will be identified via the OGAP®-Verify discovery platform of OBT, a proprietary database on membrane protein abundance, with around 7,000 cataloged proteins across cancers and healthy tissues, and will be validated through research collaboration. Any further research, development and commercialization efforts against these targets will be driven by Roche. OBT will receive up to US$36 million upfront payments from Roche and may be eligible to receive milestone payments potentially exceeding US$1 billion, plus product royalties on net sales.
Targeting the toughest tumors: how Oxford BioTherapeutics and Roche are advancing next-gen ADC therapies
Empowering immuno-oncology: Oxford BioTherapeutics develops first-in-class ADCs for difficult-to-treat cancers
Oxford BioTherapeutics was founded in Oxford, UK, in 2004. It is a clinical stage oncology company which develops immuno-oncology (IO) and antibody-drug conjugate (ADC) treatments for cancer.
Its lead clinical program, OBT076, is an ADC in Phase 1b clinical development in patients with advanced or refractory solid tumors, including gastric, bladder, ovarian and lung cancer, where CD205 is overexpressed (NCT04064359, “Safety and Preliminary Efficacy of OBT076 in Recurrent/Metastatic CD205+ Solid Tumors”, recruiting). OBT076 is composed of an antibody (Ab) that targets the CD205 protein present on immune cells promoting tumor growth, conjugated via a cleavable linker SPDB to the derivative microtubule inhibitor, DM4, which can kill them. By depleting immunosuppressive dendritic cells, OBT076 has the potential to reactivate the immune system and promote the induction and activation of T-cells to enhance the anti-tumor response.
OBT develops multiple strategic partnerships including with Boehringer Ingelheim, ImmunoGen (now part of Abbvie), Zymeworks, Amgen, WuXi, Medarex (BMS), etc.
Roche at the forefront of targeted cancer therapy with approved and emerging ADCs
Roche develops differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. It also provides in vitro diagnostics, tissue-based cancer diagnostics, and diabetes management.
Currently, Roche has 4 ADC in its pipeline, whose 2 are on the market, Polivy and Kadcyla. Polivy (Polatuzumab vedotin, RG7596) is an ADC develops with Seagen that consists of a monoclonal Ab anti-CD79b, conjugated to a potent anti-cancer agent that is selectively delivered to tumor cells. It is currently approved in combination with rituximab, cyclophosphamide, doxorubicin and prednisone in more than 95 countries worldwide, including the US and EU, for the treatment of adult patients with previously untreated diffuse large B-cell lymphoma (DLBCL). Polivy is also approved in combination with bendamustine plus rituximab in more than 100 countries worldwide for the treatment of adults with relapsed or refractory (R/R) DLBCL after one or more prior therapies. Regarding Kadcyla (trastuzumab emtansine, RG3502) is an ADC develops with ImmunoGen (acquired by AbbVie) that combines the therapeutic effect of trastuzumab (the active substance of Herceptin) with intracellular delivery of DM1, a highly potent chemotherapy agent, to specifically target HER2-positive tumors. It is indicated for high-risk patients with adjuvant HER2-positive breast cancer (HER-2+ eBC high-risk). Moreover, Roche has 2 ADC in development. First, YL211 is developed in collaboration with MediLink. It selectively targets cMET expressed on the surface of target cancer cells (solid tumors). Second, IBI3009, in partnership with Innovent, an ADC targeting DLL3 antigen with a Topo1 isomerase payload (Small Cell Lung Cancer).
Roche is exploring the ADC space more deeply through its partnership with OBT.
Transforming ADC discovery: OBT’s OGAP® platform identifies novel targets beyond conventional biomarkers
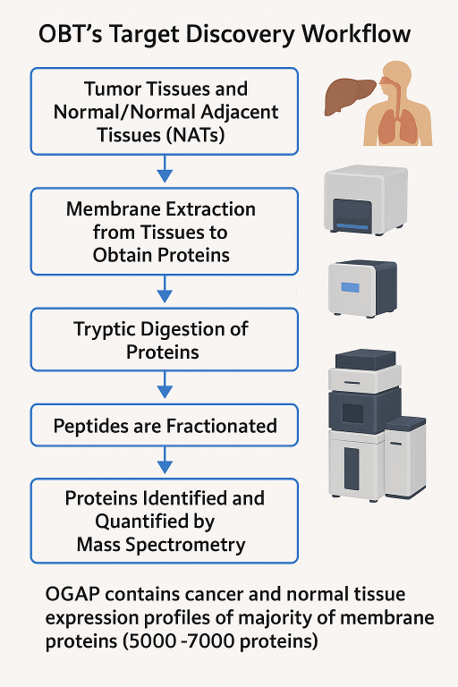
Figure 1: Workflow of OBT’s target discovery, adapted from a poster of OBT.
OBT’s proprietary Oxford Genome Anatomy Project (OGAP®) target discovery platform is based on a large proprietary cancer membrane proteomic database, with data on over 7,000 cancer cell proteins, providing highly qualified oncology targets. The company leverages its platform – OGAP® to identify first-in-class Ab based therapeutic targets by directly measuring membrane protein abundance in patient tissues using quantitative mass spectrometry.
Few cancer patients are eligible for treatment with existing ADCs because ADC-target expression on patient tumors is a major factor in patient eligibility. Furthermore, most ADCs in development target the same proteins as those already approved. With OGAP®-Verify, OBT can identify first-in-class targets to treat patients who are currently ineligible for existing treatments. The platform is able to detect protein expression levels as low as 50 copies-per-cell, which may reveal targets that were missed by mRNA analysis. Moreover, it provides insights into normal tissue expression which improves target selection and accelerates the drug target discovery process. The likelihood of success in ADC development is increased by OGAP®-Verify evaluation of factors like therapeutic index, protein abundance and benchmarking against known clinical ADC targets during target selection.
The strategic collaboration between Oxford BioTherapeutics and Roche leverages the power of the OGAP®-Verify platform, whose value is largely based on a robust intellectual property portfolio. A comprehensive set of patent families supports the scientific foundations and therapeutic applications of the platform, highlighting the breadth of novel targets and methods identified by OBT.
Identified patent families related to OGAP®-Verify
Oxford Biotherapeutics owns 26 patent families (171 documents) published between 2006 and 2019, including 21 filed between 2006 and 2011 (Table 1). The company follows a global IP strategy, with protections in the USA, Europe, Japan, and additional coverage in Canada, Australia, China, and other countries. OBT holds 11 patent families describing antibody constructs. These antibodies are capable of binding to antigens that have been observed in various types of cancer, such as:
- Lymphocyte antigen 75 (LY75): endocytic receptor to direct captured antigens from the extracellular space to a specialized antigen-processing compartment;
- Bone marrow stromal antigen 1 (BST1): lipid-anchored, bi-functional ectoenzyme that catalyzes ribonucleotide cyclisation and hydrolysis;
- Cadherin-17: calcium dependent cell adhesion proteins, they preferentially interact with themselves in a homophilic manner in connecting cells;
- Immunoglobulin superfamily member 4 (IGSF4): membrane glycoprotein with an extracellular domain homologous to those of immunoglobulin superfamily proteins. It may act as a tumor antigen recognized by activated NK or CD8+ T cells;
- Integrin beta 4: receptor for laminin, plays a critical structural role in the hemidesmosome of epithelial cells;
- Delta-like protein 3 or (DLL3): type I membrane protein, member of the Delta family;
- Matriptase stem: the protein is known to undergo a series of endoproteolytic cleavages followed by activation site autocleavage resulting in a matriptase stem which remains on the cell surface and a catalytic domain of the matriptase protein which is released into the blood;
- Mucin 13: high-molecular-weight transmembrane glycoprotein;
- Ephrin type-A receptor 7: receptor tyrosine kinase which binds promiscuously GPI-anchored ephrin-A family ligands residing on adjacent cells, leading to contact-dependent bidirectional signaling into neighboring cells.
The company also discloses several methods across 17 patent families. Six of these families describe diagnostic methods, while eight focus on cancer treatment approaches. For cancer diagnosis, assays are performed to detect polypeptides in samples from subjects, and the results are correlated with the presence or absence of cancer. These results may also be linked to a therapeutic regimen, the risk of relapse, or prognostic clinical outcomes. To evaluate colorectal cancer marker proteins (CRCMPs) in patient serum, a sandwich ELISA is performed using a biotinylated antibody, followed by a multiplex assay using Luminex Technology (with antibodies conjugated to unique Luminex magnetic microspheres — Mug beads, Luminex Corporation, Austin, TX).
For cancer treatments, a therapeutically effective amount of an antibody is administered, with a diluent or carrier. In an example, toxicity of DM1- and DM4-conjugated anti-LY75 monoclonal Ab was tested in cynomolgus monkeys. LY75_DM4 (cleavable) or LY75_DM1 (non-cleavable) was administered twice by intravenous infusion at 5 mg/kg/dose (LY75_DM4) or 10 mg/kg/dose (LY75_DM1). The treatment was well tolerated in cynomologus monkeys. All treatment-related toxicity findings were reversible following a 4-week recovery period (e.g., anemia, decrease in the blood leukocyte profile or increase in AST, CK, LDH). Most patent families describe colorectal cancer (9) or lung cancer (5).
| Patent assignee | Pub year | Patent no | Title | Object of the invention | Cancer |
| OXFORD BIOTHERAPEUTICS / BOEHRINGER INGELHEIM | 2002-06-26 | EP3736293 | Therapeutic and diagnostic target for cancer comprising dll3 binding reagents | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2008-03-06 | EP2078202 | Protein | An antibody | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2008-03-06 | EP2069793 | Identification of protein associated with hepatocellular carcinoma, glioblastoma and lung cancer | An antibody | Small cell lung |
| OXFORD BIOTHERAPEUTICS | 2008-09-04 | EP2121745 | Proteins | An antibody | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2008-09-04 | EP2121738 | Proteins | An antibody | various |
| OXFORD BIOTHERAPEUTICS / BRISTOL MYERS SQUIBB | 2009-02-12 | EP2185933 | Matriptase protein and uses thereof | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2009-12-17 | EP2297580 | Antibody against Ephrin Type-A receptor 7 for treatment of bladder cancer | An antibody | various |
| OXFORD BIOTHERAPEUTICS / BRISTOL MYERS SQUIBB | 2010-11-11 | US20100285017 | Matriptase protein and uses thereof | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2013-01-03 | EP2726094 | Therapeutic and diagnostic target | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2014-08-13 | EP2765140 | Cadherin-2 or Mucin-13 binding molecules for cancer treatment | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2015-04-16 | EP3055331 | Conjugated antibodies against ly75 for the treatment of cancer | An antibody | various |
| OXFORD BIOTHERAPEUTICS | 2006-10-04 | US20090238833 | Protein | A method of treating or preventing | Colorectal / Lung |
| OXFORD BIOTHERAPEUTICS | 2006-10-04 | US20090238830 | Protein | A method of treating or preventing | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2009-09-24 | US20090238834 | Identification of protein associated with hepatocellular carcinoma, glioblastoma and lung cancer | A method of treating or preventing | HCC / Glioblastoma / Lung |
| OXFORD BIOTHERAPEUTICS | 2010-04-22 | US20100098628 | Proteins | A method of treating | various |
| OXFORD BIOTHERAPEUTICS | 2010-04-22 | US20100098627 | Proteins | A method of treating | Colorectal / Kidney / Lung / Pancreatic |
| OXFORD BIOTHERAPEUTICS | 2010-04-22 | US20160175437 | Proteins | A method of treating | various |
| OXFORD BIOTHERAPEUTICS | 2010-11-11 | US20100284908 | Proteins | A method of treating | various</td |
| OXFORD BIOTHERAPEUTICS | 2019-10-10 | US20190310255 | Proteins | A method of treating | various |
| OXFORD BIOTHERAPEUTICS | 2006-07-19 | US20090169575 | Proteins | A method of diagnosing | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2006-09-06 | US20090208507 | Protein isoforms and uses thereof | A method of diagnosing | no |
| OXFORD BIOTHERAPEUTICS | 2007-12-13 | EP2074426 | Proteins | A method of diagnosing | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2008-01-31 | EP2121747 | New protein isoforms and uses thereof | A method of diagnosing | no |
| OXFORD BIOTHERAPEUTICS | 2008-03-06 | EP2078203 | Protein | A method of diagnosing | Colorectal / Lung |
| OXFORD BIOTHERAPEUTICS / IBM | 2011-08-11 | US20140024048 | Protein | A method of diagnosing | Colorectal |
| OXFORD BIOTHERAPEUTICS | 2007-11-14 | EP2200636 | Protein | A composition | various |
Table 1: patent families owned by Oxford Biotherapeutics and describing OGAP®-Verify.
The collaboration between Oxford BioTherapeutics and Roche represents a significant step forward in the evolution of antibody-drug conjugate development. By harnessing the power of OBT’s OGAP®-Verify platform to uncover novel, first-in-class targets, this partnership aims to expand treatment options for patients with cancers currently underserved by existing therapies. Furthermore, beyond the scientific and technological complementarity of the two companies, this alliance is supported by a solid intellectual property base, with a portfolio of strategic patents protecting the targets, antibodies, and associated methods. This IP dimension not only reinforces the value of the OGAP® platform but also ensures freedom of operation and sustainable differentiation in the ADC market. Together, OBT and Roche are paving the way for more effective and personalized cancer treatments.
Oncology treatment innovation is one of KnowMade’s areas of healthcare expertise that you may need for your IP and R&D projects. For more information, please contact us.
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Fabienne Massa, PhD., works for Knowmade in the field of Biotechnology and Life Sciences. She holds a PhD in Molecular and Cellular Biology from the IPMC (Sophia Antipolis, France). She also holds a Master of Business Management from IAE (Nice, France) and she previously worked in the pharmaceutical industry.
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Foo
