SOPHIA ANTIPOLIS, France – March 22, 2023 │The Quarterly Report for the Q4 2022 Therapeutic mRNA Patent Monitor is now available! This report covers all aspects of mRNA design, delivery, manufacturing, storing, mRNA-based vaccines, and mRNA-based therapeutics. Let’s focus on one of the most dynamic segments.
KnowMade has developed expertise in the field of therapeutic mRNA, allowing a comprehensive view of this disruptive technology through landscape reports on mRNA vaccines, mRNA cancer therapies, and self-amplifying RNA vaccines. In this ever-changing context, it is essential to monitor patenting activities related to therapeutic mRNA to be aware of weak signals that could hint at the future of this technology, and to understand the intellectual property position and strategy of the players.
One of the bottlenecks for mRNA therapies is delivery efficiency. Indeed, mRNAs are inherently highly unstable and vulnerable to degradation by RNA-cutting ribonuclease enzymes when administered into the human body. Moreover, naked mRNA molecules face huge challenges to diffuse across the cell membrane due to their hydrophilicity and negative charge, as well as avoiding endo-lysosomal degradation and reaching the desired tissue or organ. To improve mRNA therapies, both the technology and the delivery system must be advanced.
Delivery is a key in patenting activity
According to KnowMade’s latest quarterly mRNA patent monitoring report (see here), a large part of today’s patent activity is focused on improving mRNA delivery while developing targeted strategies. Indeed, 40% of the new patent applications are strictly related to mRNA carriers, with 30% of them related to LNP. This tendency is also observed in the recent patent landscape on self-amplifying RNA for vaccination, as LNP have the largest number of pending patent applications related to vaccination for SARS-CoV2 and cancerous disorders (see here). In Q4 2022, LNP are still the most investigated delivery systems (see figure below).
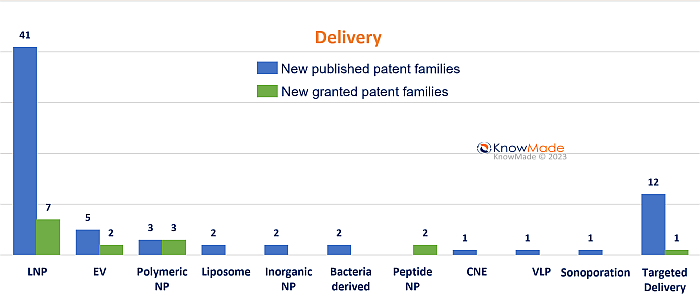
Number of patent families published or granted in Q4 2022 segmented by delivery system.
LNPs are derivatives of the phospholipid-based liposomes first generated in the 1960s. They are composed of four key components: structural lipids, cholesterol, ionizable cationic lipids, and stealth lipids. Structural lipids are mainly neutrally charged phospholipids, cholesterol improves LNP stabilization and properties (membrane fluidity, elasticity, and permeability), cationic lipids allow the loading of mRNA which is a negative molecule, and stealth lipids, mainly polyethylene glycol (PEG) polymer–conjugated lipids, are added to reduce immunogenicity (Rohner et al., 2022). Currently, LNP compositions are being improved for different purposes, such as increasing LNP stability, reducing nanoparticle immunogenicity (mainly for non-vaccine mRNA), or targeting specific tissues or cells.
Recent surge of companies engaging in LNP-mRNA formulation
Platform technologies are developed by established companies as well as startup firms to manufacture LNP-mRNA formulations. For example, INVENTAGE LAB, a Korean company founded in 2015, published its first patent application related to therapeutic mRNA (KR10-2022-0145788) in Q4 2022, which describes a method for producing LNP using a microfluidic apparatus (see figure below). This invention is based on INVENTAGE LAB’s expertise in microfluidics and allows for the development of the IVL-GeneFluidic® platform to encapsulate mRNA. This platform allows for a uniform LNP size, which is a critical feature for LNP body-distribution, immunogenicity, and production yield.
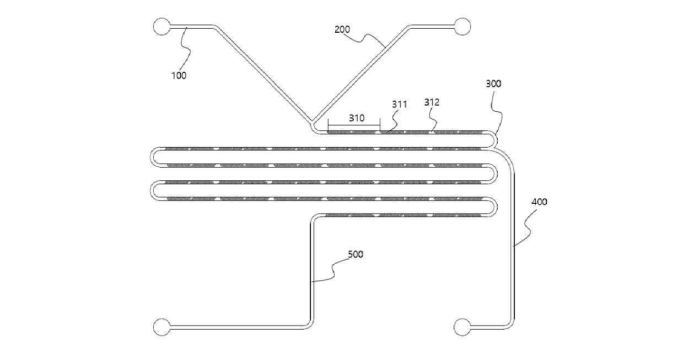
Schematic representation of preparation apparatus for preparing lipid nanoparticles from INVENTAGE LAB
The manufacturing apparatus includes two channels (100 and 200) linked to a stirring channel (300) comprising a mixing module repeatedly formed (310) for nucleic acid/ionized lipid binding. A third channel (400) allows for the combination of other lipids for LNP formulation (from KR10-2022-0145788).
A second example from Q4 2022 is LEON NANODRUGS, a German company founded in 2010 which also published its first patent application related to therapeutic mRNA (WO2022/234050). The company utilizes its proprietary nano technology platform NANOus® for medium- to large-scale manufacturing of LNP-encapsulated APIs, such as mRNA vaccines. According to LEON NANODRUGS, the company has recently completed the development of its innovative reactor for LNP production.
The patent-monitoring activity shows that LNP-based delivery is also a core technology for new startup firms (see figure below). Among companies identified in Q4 2022, NANOVATION THERAPEUTICS develops a platform comprising in-house synthetic organic chemistry, mRNA synthesis, and formulation in LNP; THERNA THERAPEUTICS is developing LNPs for siRNA and mRNA; and WESTGENE BIOPHARMA plans to develop the two core technologies of mRNA sequence and delivery system. In Q4 2022, this company completed a $21 million Series A Round to advance its portfolio of nearly 20 mRNA candidates and published its first patent application related to ionizable lipids for LNPs.

New startup firms identified in Q4 2022
In Q4 2022, six new companies developing therapeutic mRNA have published their first patent application. The innovative tendency towards LNP development and alternative RNA designs is confirmed by the technologies developed by these new startups. Indeed, four of them are focused on LNP for mRNA delivery (NANOVATION, THERNA, WEST GENE BIOPHARMA, and RENAGADE), and two are focused on alternative designs for mRNA (CHIMERON BIO, which develops sa-mRNA, and CIRCODE, which develops Circ RNA). These new companies were all founded in North America or Asia; none are from the European area. (From Q4 2022 KnowMade’s patent monitor reports).
Press contact
contact@knowmade.fr
Le Drakkar, 2405 route des Dolines, 06560 Valbonne Sophia Antipolis, France
www.knowmade.com
About the author
Elodie Bovier, PhD., works at KnowMade as a Patent Analyst in the field of Biotechnology and Life Sciences. She holds a PhD in genetic and molecular biology from the Paris Sud University. She also holds the Industrial Property International Studies Diploma (in Patent and Trademark & Design Law) from the CEIPI (Strasbourg, France).
About KnowMade
KnowMade is a technology intelligence and IP strategy consulting company specialized in analyzing patents and scientific publications. The company helps innovative companies, investors, and R&D organizations to understand competitive landscape, follow technological evolutions, reduce uncertainties, and identify opportunities and risks in terms of technology and intellectual property.
KnowMade’s analysts combine their strong technology expertise and in-depth knowledge of patents with powerful analytics tools and methodologies to turn patent information and scientific literature into actionable insights, providing high added value reports for decision makers working in R&D, innovation strategy, intellectual property, and marketing. Our experts provide prior art search, patent landscape analysis, freedom-to-operate analysis, IP due diligence, and monitoring services.
KnowMade has a solid expertise in Compound Semiconductors, Power Electronics, Batteries, RF Technologies & Wireless Communications, Solid-State Lighting & Display, Photonics, Memories, MEMS & Sensors, Semiconductor Packaging, Medical Devices, Medical Imaging, Microfluidics, Biotechnology, Pharmaceutics, and Agri-Food.
